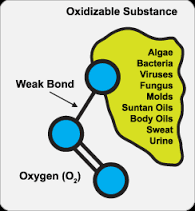
Disinfection
Bacteria, viruses, protozoa, worms and fungi are present in the aquatic environment. Water-borne and water-associated communicable diseases caused by pathogenic organisms include bacillary and amoebic dysentery, cholera, typhoid and paratyphoid fever, bacterial gastroenteritis, leptospirosis, infectious hepatitis, poliomyelitis, and diarrhea and enteritis caused by enteroviruses and protozoa.
Because of problems in detecting disease-causing bacteria and viruses, the microbial safety of water is determined indirectly. Indicator organisms are used to determine the extent of bacterial contamination. If it can be shown (using indicator organisms) that fecal contamination of the water has occurred; then, pathogenic organisms may also be present. The most commonly used bacterial indicators are coliforms and fecal streptococci (fecal coliforms).
High counts of coliform bacteria, especially fecal coliform, indicate the presence of animal wastes, which may also support pathogenic organisms. Such waters are unsuitable for domestic, recreational, agricultural, and some industrial applications.
The CT (concentration-times-time) product has been established to define the disinfection efficiency of various chemical disinfectants. The CT product is based on several individual experiments which are carried out using different disinfectant concentrations under otherwise identical experimental conditions.
Disinfection & Ozonation
Ozone, the most powerful of oxidants, is far superior to chlorine, chlorine dioxide, or chloramines for the inactivation of all types of waterborne pathogens. Regardless of the pathogen type, the concentration-times-time (CT) values associated with ozone are orders of magnitude lower than those associated with any other oxidant.
This table provides summary of CT value ranges for 99% inactivation of various micro-organisms by disinfectants at 5 deg. C. From the CT values, it is evident that ozone is the most efficient disinfectant for all types of micro-organisms.
Bacteria
E. Coli and Salmonella are two of the most common types of bacteria we are exposed to, and both are very sensitive to inactivation by ozone. The oxidation and bacterial inactivation reactions which occur upon exposure with ozone occur very quickly, making ozonation very effective against bacteria.
Viruses
Viruses are generally more resistant to ozone than vegetative bacteria. Experimental evidence indicates gram-negative bacteria to be on the order of 10-fold more susceptible than viruses to ozone. For virus inactivation the CT for chlorine is approximately 6 times larger than the CT for ozone.
Parasites
Giardia and Cryptosporidium are protozoa; their life cycles consist of two forms: fragile trophozoites and cysts. Trophozoites are too fragile to live in the environment. They stay in the infected host intestine, where they multiply and encyst. Cysts, the only form found in the environment, are discharged with feces and disseminate the species. Thus, they always enter waterways through the fecal route and their subsequent ingestion may lead to infection.
Today G. lamblia is widely recognized as the most frequently identified cause of waterborne disease. The disinfectant concentration (CT) for G. lamblia and G. muris are 0.53 and 1.94 mg.min/L at 5o C, and 0.17 and 0.27 mg.min/L at 25o C for 99 percent inactivation at pH 7. These CT values for inactivation of G. lambia with free chlorine, the chemical disinfectant of choice for many years, are approximately 100 times larger than those reported for ozone.
The USEPA (United States Environmental Protection Agency) stated in a report in 1989 that the CT requirements for ozone inactivation of 99.9% of G. lambia also achieved a 99.99% inactivation of viruses. The assumption being made was that if conditions for ozone inactivation of G. lambia cysts were adequate, viruses would then also be inactivated to the required level.
Cryptosporidium causes cryptosporidiosis if ingested, an intestinal disease which makes healthy people very sick, and can kill people whose immune systems have been compromised, as happened in Milwaukee Wisconsin during the early 1990’s. Cryptosporidium is very difficult to remove by filtration because of its small size. It is also resistant to chemical disinfection; both chlorine and chloramine have virtually no effect. It is possible that 0.5 log inactivation can be achieved but only after ‘days’ of contact with chlorine. Only chlorine dioxide and ozone have been proven to be effective for the chemical inactivation of Cryptosporidium parvum, and as might be expected ozone is an order of magnitude more effective than chlorine dioxide. Around 3-6 times more ozone is required for the inactivation of Cryptosporidium than for Giardia lamblia cysts. Ozone is therefore the only disinfectant capable of inactivating this microorganism without forming additional halogenated organic by-products of regulatory concern.